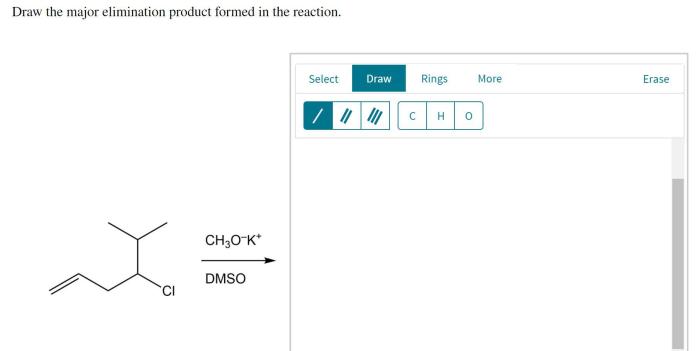
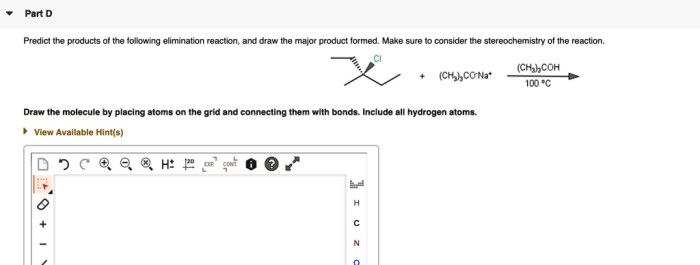
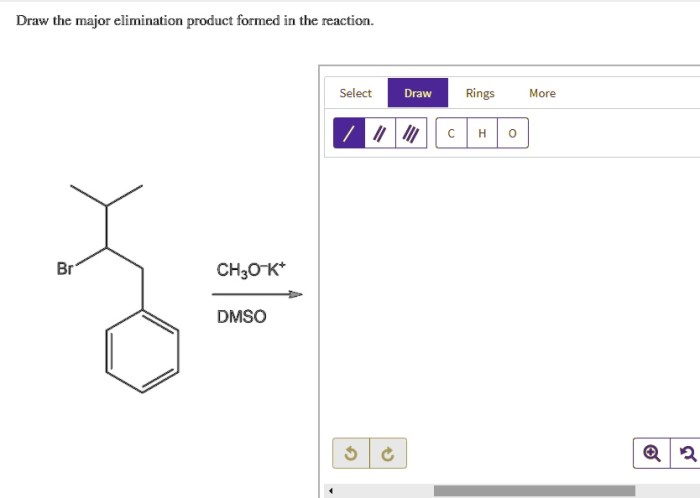
Draw the major elimination product formed in the reaction: A Comprehensive Guide. Elimination reactions are a fundamental class of organic reactions that involve the removal of two atoms or groups of atoms from a molecule, resulting in the formation of a new double or triple bond.
Understanding the factors that influence the formation of the major elimination product is crucial for predicting the outcome of these reactions and harnessing their synthetic potential.
This guide delves into the intricacies of elimination reactions, exploring the role of substrate structure, reaction conditions, and catalyst or base strength in determining the regio- and stereoselectivity of the reaction. It also highlights the diverse applications of elimination reactions in organic synthesis, polymer chemistry, and various industrial processes.
Major Elimination Products in Organic Reactions: Draw The Major Elimination Product Formed In The Reaction
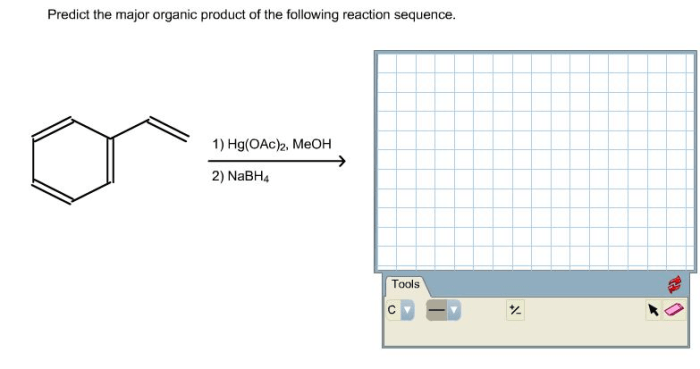
In organic chemistry, elimination reactions involve the removal of two substituents from adjacent carbon atoms, resulting in the formation of a double or triple bond. The major elimination product is the most prevalent product formed under specific reaction conditions.
Examples of elimination reactions include the E2 (bimolecular elimination) and E1 (unimolecular elimination) reactions, which produce alkenes and alkynes, respectively.
Factors Influencing Product Formation, Draw the major elimination product formed in the reaction
The major elimination product formed in a reaction is influenced by several factors:
- Substrate structure:The nature of the substrate, including the functional groups and substitution patterns, plays a crucial role in determining the major product.
- Reaction conditions:Temperature, solvent, and reaction time can affect the product distribution. Higher temperatures favor E2 reactions, while lower temperatures promote E1 reactions.
- Catalyst or base strength:Strong bases favor E2 reactions, while weak bases or acids promote E1 reactions.
Regioselectivity and Stereoselectivity
Regioselectivity refers to the preference for a particular double or triple bond to form, while stereoselectivity involves the preferential formation of a specific stereoisomer.
Regioselectivityis influenced by factors such as the stability of the carbocation intermediate and the steric hindrance around the reaction site.
Stereoselectivityis governed by factors such as the geometry of the starting materials, the presence of chiral catalysts, and the reaction mechanism.
Applications of Elimination Reactions
Elimination reactions find wide applications in organic synthesis:
- Alkene and alkyne synthesis:Elimination reactions are commonly used to prepare alkenes and alkynes, which are important building blocks for various organic compounds.
- Polymer chemistry:Elimination reactions are involved in the synthesis of polymers, such as polyethylene and polypropylene.
- Industrial applications:Elimination reactions are employed in the production of a variety of industrial chemicals, including ethylene, propylene, and styrene.
FAQ Resource
What is the major elimination product?
The major elimination product is the product that is formed in greater quantity than any other elimination product in a given reaction.
What factors influence the formation of the major elimination product?
The formation of the major elimination product is influenced by the substrate structure, reaction conditions, and catalyst or base strength.
What is regioselectivity?
Regioselectivity refers to the preference for the formation of one double or triple bond over another in an elimination reaction.
What is stereoselectivity?
Stereoselectivity refers to the preference for the formation of one stereoisomer over another in an elimination reaction.