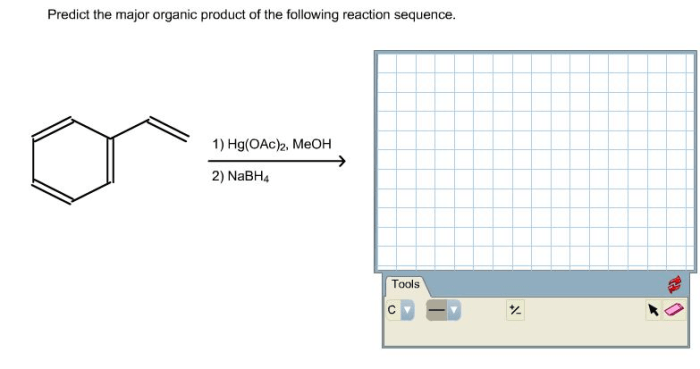
Condensed structural formula for 1 4 dichlorocyclohexane – Unveiling the intricacies of 1,4-dichlorocyclohexane, this exploration delves into its condensed structural formula, providing a comprehensive understanding of its molecular makeup and chemical characteristics.
As a cyclic hydrocarbon bearing two chlorine atoms, 1,4-dichlorocyclohexane exhibits a unique arrangement of atoms represented by its condensed structural formula: C6H10Cl2. This formula succinctly conveys the molecular composition, highlighting the presence of six carbon atoms, ten hydrogen atoms, and two chlorine atoms.
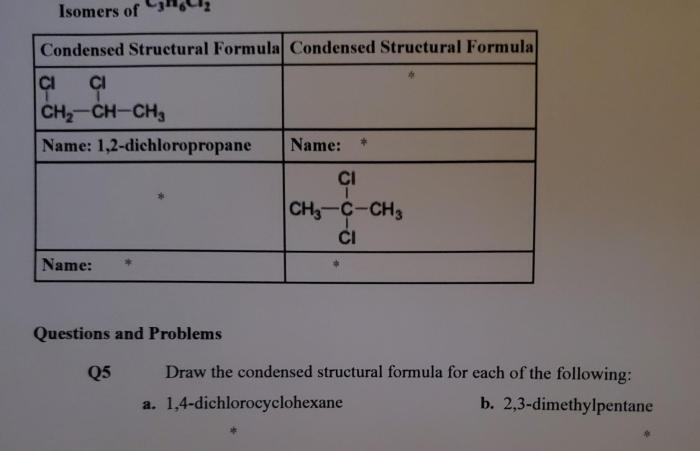
1. Structural Formula
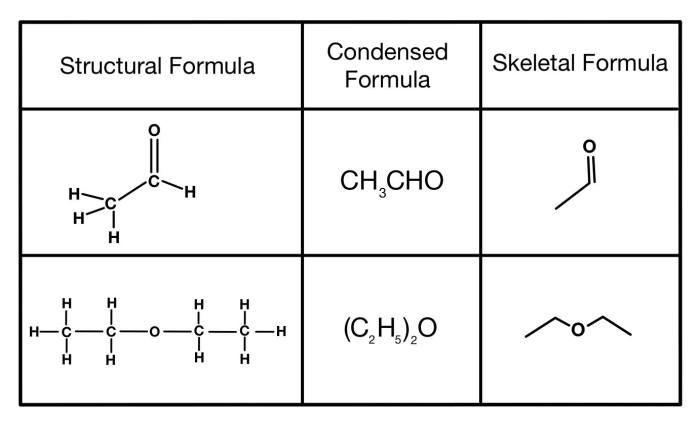
The condensed structural formula for 1,4-dichlorocyclohexane is C 6H 10Cl 2. It can be represented visually as:
The IUPAC nomenclature for 1,4-dichlorocyclohexane is 1,4-dichloro-cyclohexane.
2. Chemical Properties
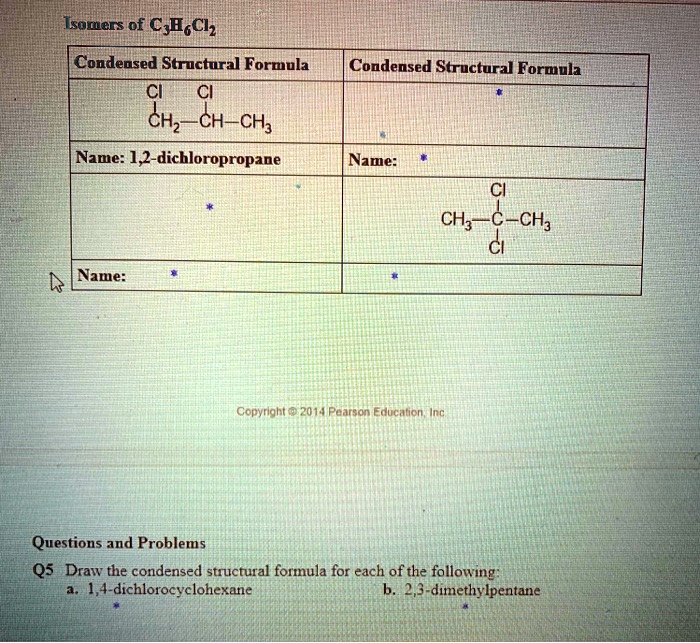
Physical Properties
- Boiling point: 208-210 °C
- Melting point: -40 °C
- Density: 1.23 g/cm 3
Chemical Properties
1,4-Dichlorocyclohexane is a reactive compound due to the presence of two chlorine atoms. It undergoes nucleophilic substitution reactions, where the chlorine atoms are replaced by other nucleophiles.
Examples of reactions involving 1,4-dichlorocyclohexane include:
- Reaction with sodium hydroxide to form 1,4-cyclohexanediol
- Reaction with ammonia to form 1,4-cyclohexanediamine
- Reaction with alcohols to form 1,4-cyclohexyl ethers
3. Production Methods: Condensed Structural Formula For 1 4 Dichlorocyclohexane
1,4-Dichlorocyclohexane is produced by the following methods:
Chlorination of Cyclohexane, Condensed structural formula for 1 4 dichlorocyclohexane
This is the most common method, where cyclohexane is reacted with chlorine gas in the presence of a catalyst.
Addition of Hydrogen Chloride to 1,3-Butadiene
1,3-Butadiene is reacted with hydrogen chloride in the presence of a catalyst to form 1,4-dichlorocyclohexane.
Advantages and Disadvantages
The advantages and disadvantages of each method are:
| Method | Advantages | Disadvantages |
|---|---|---|
| Chlorination of Cyclohexane | High yield | Formation of byproducts |
| Addition of Hydrogen Chloride to 1,3-Butadiene | Less byproduct formation | Lower yield |
4. Safety Considerations

Hazards
- 1,4-Dichlorocyclohexane is a toxic substance.
- It is harmful if swallowed, inhaled, or absorbed through the skin.
- It can cause irritation to the eyes, skin, and respiratory tract.
Handling and Storage
1,4-Dichlorocyclohexane should be handled and stored with care. It should be stored in a cool, dry place away from sources of heat and ignition. It should be handled in a well-ventilated area and appropriate personal protective equipment should be worn.
Environmental Impact
1,4-Dichlorocyclohexane is a persistent organic pollutant (POP). It can accumulate in the environment and pose a threat to wildlife. Mitigation strategies include proper disposal of waste containing 1,4-dichlorocyclohexane and reducing its use in industrial processes.
FAQ Corner
What is the IUPAC name for 1,4-dichlorocyclohexane?
1,4-Dichlorocyclohexane
What are the physical properties of 1,4-dichlorocyclohexane?
Boiling point: 172-174 °C, Melting point: -22.6 °C, Density: 1.17 g/cm³
What are the common uses of 1,4-dichlorocyclohexane?
Solvent, intermediate in the production of other chemicals, and a precursor for the synthesis of pharmaceuticals