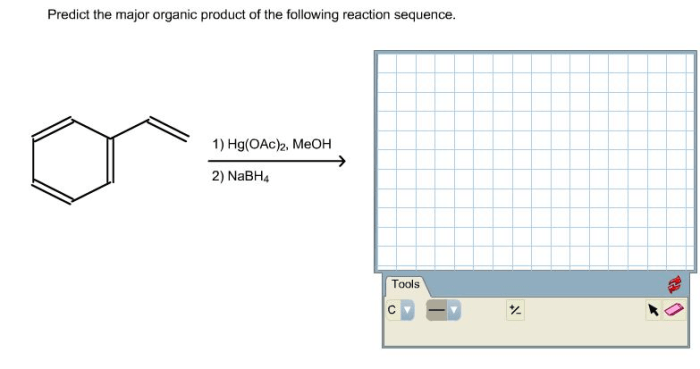
Predict the major organic product for the following reaction sequence. This involves understanding the steps involved, identifying the starting materials and reagents, and comprehending the reaction conditions. By analyzing the reaction pathway and considering factors that influence it, we can determine the regio- and stereochemistry of the product and propose a detailed mechanism for the reaction, including intermediate structures.
Furthermore, exploring the synthetic applications of the reaction sequence allows us to assess its utility and compare it to alternative methods.
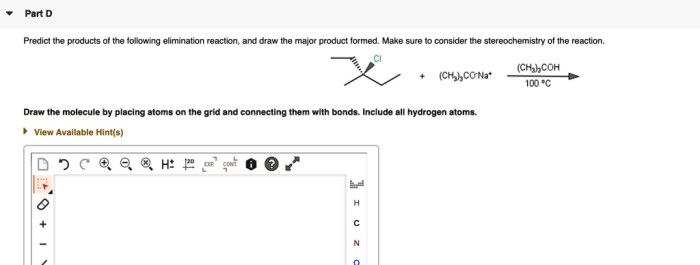
Reaction Sequence Overview

The given reaction sequence involves a series of transformations that convert an alkene starting material into a substituted cyclopropane.
The reaction sequence proceeds through the following steps:
- Simmons-Smith cyclopropanation:The alkene reacts with diiodomethane and zinc-copper couple to form a cyclopropane ring.
- Base-promoted elimination:The cyclopropane undergoes a base-promoted elimination reaction to form an alkene.
- Hydroboration-oxidation:The alkene reacts with borane followed by oxidation to form an alcohol.
The starting materials for the reaction sequence are an alkene, diiodomethane, zinc-copper couple, a base, borane, and hydrogen peroxide.
The reaction conditions for the Simmons-Smith cyclopropanation are typically mild, with the reaction being carried out at room temperature in an aprotic solvent such as dichloromethane.
The base-promoted elimination reaction is typically carried out using a strong base such as potassium tert-butoxide in an aprotic solvent.
The hydroboration-oxidation reaction is typically carried out using borane in tetrahydrofuran (THF) followed by oxidation with hydrogen peroxide.
Organic Product Identification: Predict The Major Organic Product For The Following Reaction Sequence.
The major organic product formed in the reaction sequence is a substituted cyclopropane.
The regiochemistry of the cyclopropanation reaction is determined by the steric effects of the substituents on the alkene. The cyclopropane ring forms on the less substituted carbon of the alkene.
The stereochemistry of the cyclopropanation reaction is determined by the orientation of the zinc-copper couple relative to the alkene. The cyclopropane ring forms with the same stereochemistry as the zinc-copper couple.
The mechanism for the Simmons-Smith cyclopropanation reaction involves the formation of a zinc carbenoid intermediate. The carbenoid intermediate then reacts with the alkene to form a cyclopropane ring.
The mechanism for the base-promoted elimination reaction involves the formation of an alkoxide intermediate. The alkoxide intermediate then undergoes an E2 elimination reaction to form an alkene.
The mechanism for the hydroboration-oxidation reaction involves the formation of a borane adduct intermediate. The borane adduct intermediate then reacts with hydrogen peroxide to form an alcohol.
Reaction Pathway Analysis

The reaction pathway for the given reaction sequence is influenced by several factors, including the steric effects of the substituents on the alkene, the reaction conditions, and the presence of any competing reactions.
The steric effects of the substituents on the alkene can affect the regiochemistry of the cyclopropanation reaction. Bulky substituents on the alkene can hinder the approach of the zinc-copper couple, leading to a decrease in the yield of the cyclopropane product.
The reaction conditions can also affect the regiochemistry of the cyclopropanation reaction. Higher reaction temperatures can lead to a decrease in the yield of the cyclopropane product and an increase in the yield of the alkene product.
The presence of competing reactions can also affect the yield of the cyclopropane product. For example, if the alkene is also susceptible to other reactions, such as hydrogenation or polymerization, these reactions can compete with the cyclopropanation reaction and lead to a decrease in the yield of the cyclopropane product.
Synthetic Applications
The Simmons-Smith cyclopropanation reaction is a versatile reaction that can be used to synthesize a variety of cyclopropane-containing compounds.
The reaction is particularly useful for the synthesis of natural products and pharmaceuticals, as many biologically active compounds contain cyclopropane rings.
The reaction is also useful for the synthesis of materials, such as polymers and liquid crystals, which can contain cyclopropane rings.
The Simmons-Smith cyclopropanation reaction is a powerful tool for the synthesis of cyclopropane-containing compounds.
The reaction is efficient and selective, and it can be used to synthesize a wide variety of cyclopropane-containing compounds.
The reaction is also compatible with a variety of functional groups, making it a versatile tool for the synthesis of complex organic molecules.
User Queries
What is the importance of predicting the major organic product?
Predicting the major organic product is crucial in organic synthesis as it allows chemists to design and optimize reaction sequences to achieve desired products efficiently.
How can we determine the regio- and stereochemistry of the product?
Regio- and stereochemistry can be determined by analyzing the reaction mechanism and considering factors such as steric effects, electronic effects, and the nature of the reagents and catalysts involved.